SARS-CoV-2 Antigen rapid test
virus in humans from nasopharyngeal samples.
Rápido
Preciso
Fácil
RAPID, PRECISE AND EASY TO USE TEST
biotical SARS-CoV-2 Ag card test is a very valuable tool in terms of cost and time compared to other diagnostic methods, with a much earlier detection window than a serological antibody test. It is able to identify an active infection in under 10 minutes and with no need for laboratory equipmen.
Our test’s monoclonal antibody has high specificity to the SARS-CoV-2 antigen, limiting the possibility of false positives.
biotical SARS-CoV-2 Ag card test is manufactured in Spain by Biotical Health S.L.U, under European Regulation 98/79/EC on In Vitro Diagnostic Medical Devices, as well as the ISO13485 certification.
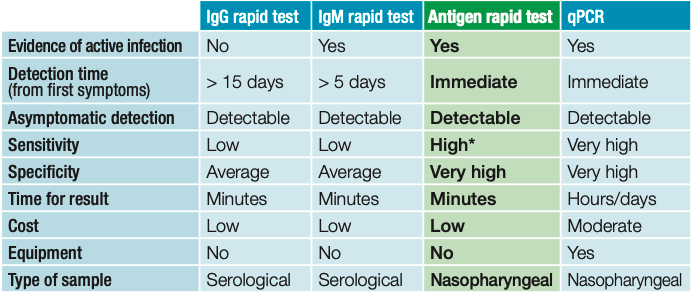
| IgG rapid test | IgM rapid test | Antigen rapid test | qPCR | |
|---|---|---|---|---|
|
Evidence of active infection |
No | Yes | Yes | Yes |
|
Detection time (from first symptoms) |
> 15 days | > 5 days | Inmediate | Inmediate |
|
Asymptomatic detection |
Detectable | Detectable | Detectable | Detectable |
|
Sensitivity |
Low | Low | High* | Very high |
|
Specificity |
Average | Average | Very high | Very high |
|
Time for result |
Minutes | Minutes | Minutes | Hours/days |
| Cost | Low | Low | Low | Moderate |
| EquipamienT | No | No | No | Yes |
| Type of sample | Serological | Serological | Nasopharyngeal | Nasopharyngeal |
What is the SARS-CoV-2 virus?
The SARS-CoV-2 virus is a new type of coronavirus responsible for the disease ca- lled COVID19.
COVID19 presents with a very wide picture of symptoms, among which are fever, fatigue, sore throat, headache, dyspnoea, diarrhoea, loss of taste and smell, as well as circulatory and organic complications of various types. SARS-CoV-2
is considered a respiratory virus that potentially causes pneumonia.
SARS-CoV-2 has demonstrated a high level of human-to-human transmission due to its long incubation period (from two to twelve days), the ability to occur asymptomatically in certain individuals and to remain active on surfaces.

The virus is spread by drops of saliva when talking, coughing or sneezing over short distances and to a lesser extent by aerosols.
Other routes of transmission such as faecal contamination are possible.
The virus enters the nasopharyngeal cavity where it enters into the epithelial cells and shows its highest viral load in the first days, replicating and accessing other cells in the body with specific receptors as the disease progresses.
PRODUCT MANUFACTURED AND DEVELOPED IN SPAIN

PRODUCT MANUFACTURED AND DEVELOPED IN SPAIN 
biotical health
SARS-CoV-2 Ag card
Procedure
1º
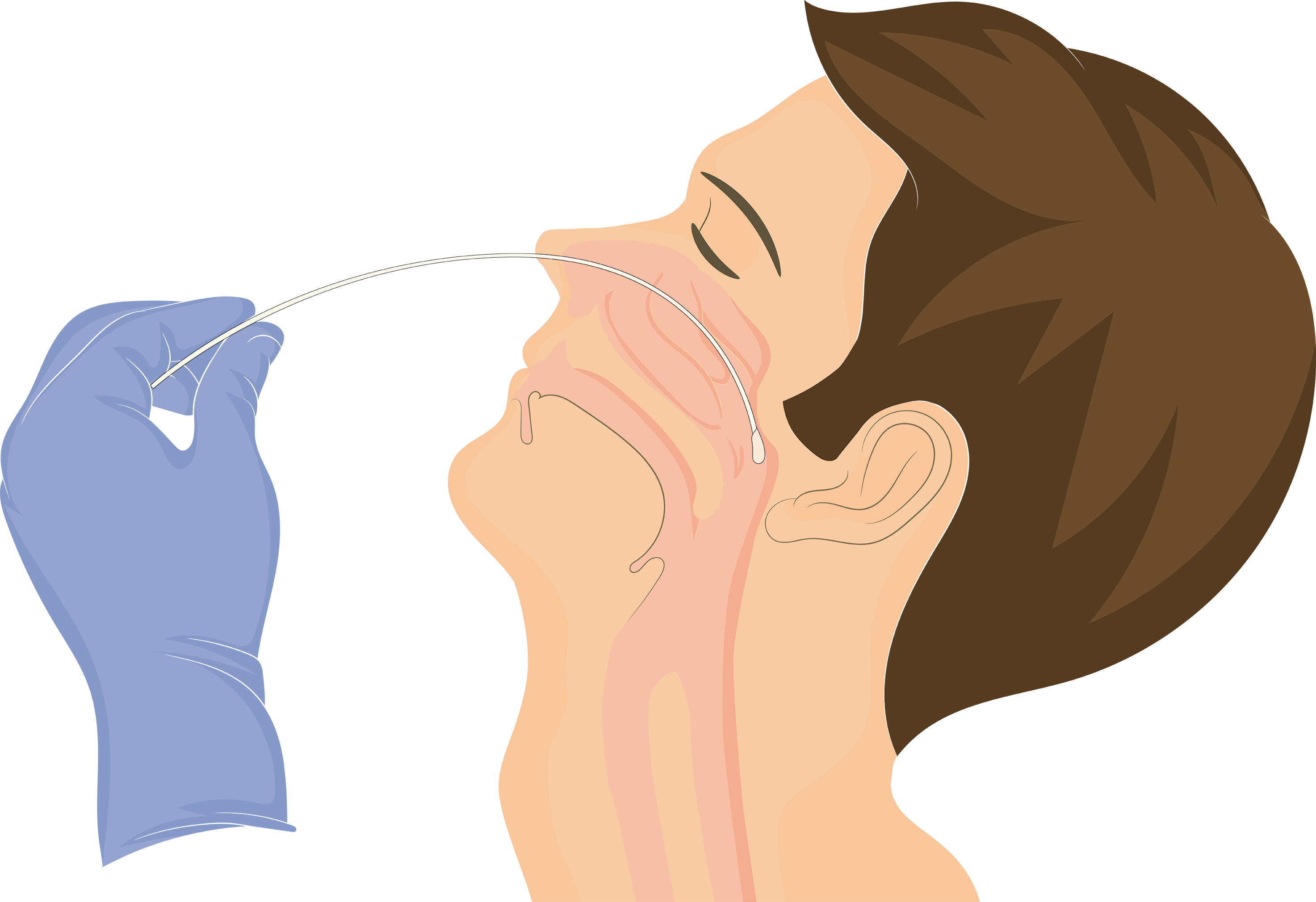
Remove the sterile swab from its container and insert it until it makes contact with the nasopharynx, then gently rotate the swab against the nasal wall to capture both cells and snot.
2º

Put the swab into the tube and rotate it for 1 minute to extract the liquid.
3º

Read the results after 10 minutes
NEGATIVE
POSITIVE
INVALID
INVALID
biotical SARS-CoV-2 Ag card Vs qPCR technique
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Mean value Intervalo de confianza |
92,9% 76,5 – 99,1% |
99,6% 97,6 – 100,0% |
96,3% 81,0 – 99,9% |
99,1% 97,0 – 99,9% |



| SARS-CoV-2 biotical Kit | Test card in sealed container |
Swab in sterile container | Test tubes and pipettes | Diluent | Extra quality control |
|---|---|---|---|---|---|
| Contents of the case | 25 uds. | 25 uds. | 25 + 25 uds. | Incluido | Control Positivo incluido |


Do you need information?
Phone number
(+34) 91 677 43 08
WEB
Address
Biotical Health, S.L.U.
C/ Sierra de Guadarrama, 1.
28830 San Fernando de Henares (Madrid) España
Important
This is a product classified as “In Vitro Diagnosis”, it is not a “Self Diagnosis” product, therefore it cannot be sold to individuals, but to Hospitals, Health Centers, Clinics, Laboratories, Nursing Homes, Services Public, Risk Prevention Mutuals or Companies, provided that the guidelines established by the Ministerial order published in the BOE of April 14, 2020 are met, which establishes that “the performance of diagnostic tests for the detection of of COVID-19 to those cases in which there is a previous prescription by a doctor and they adjust to criteria established by the competent health authority “